Links to some data tables and standard thermodynamic tables and databases:
- NIST Chemistry WebBook.
- Wired Chemistry – Thermodynamic Data.
- FACT Compound Database – Compound Web.
- Free Energy Table – Hmolpedia.
- ATcT Tables.
Links to some published thermodynamic data tables:
- Cp and Entropies of Organic Cmpds in the Condensed Phase, Vol 2. J. Phys. Chem. Ref. Data, Vol 19(4), 1990: Cp_Entropies_Organic_Cmpds_V2.pdf
- Pchem_Life_Sciences_1st_Ed_Atkins_Data_Tables.pdf
Castellan’s Chemical Thermodynamic Properties at 298.15 K Table (AV.1)
 Acidity Constants
Acidity Constants



Tinoco’s Standard Reduction Electrode Potentials at 298.15 K Table (7.1)

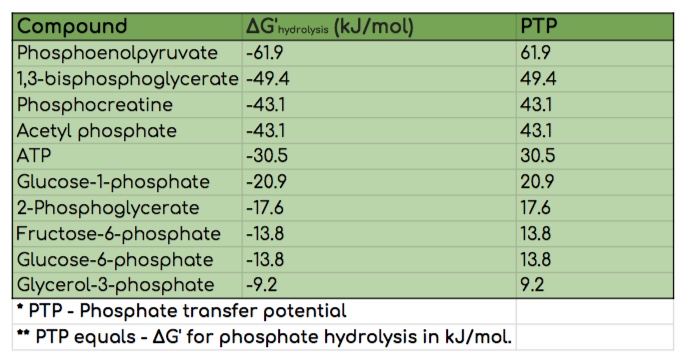
Phosphate Transfer Potentials (PTP)
REDOX Midpoint Potentials for some Biological Reactions:

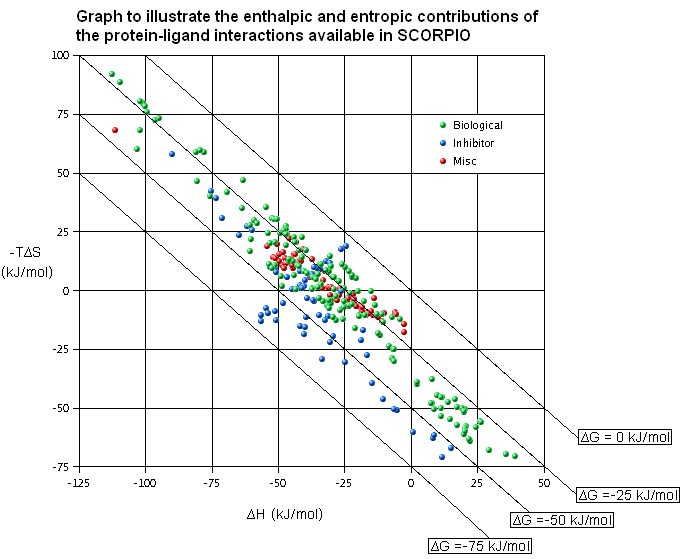
SCORPIO (FREE online repository of protein-ligand complexes which have been structurally resolved and thermodynamically characterised):
Additional Tables and Data from Tinoco (5th Ed)
And lets no forget a hard pc of data to find… and I am sure students taking Exam 1 in the Fall 2019 will care about…. 😉

Tinoco’s textbook 5th Ed.
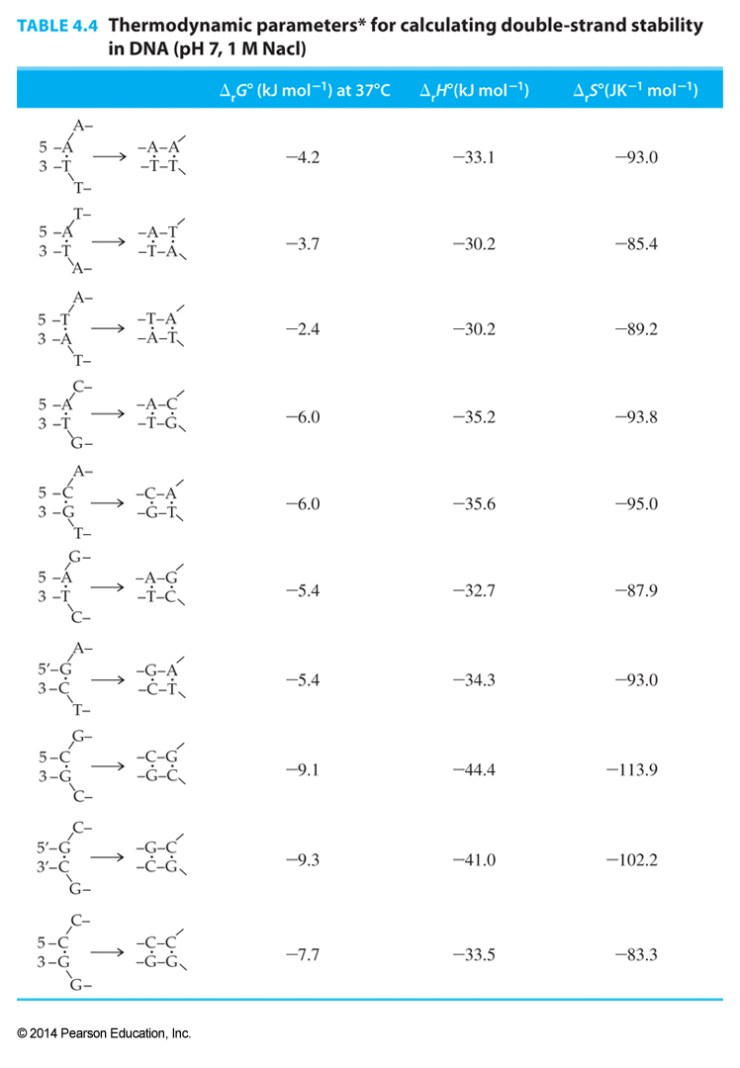
The initial interaction of two strands contributes a loss of entropy and an unfavorable free energy that must be included in calculations:
initiation Gibbs free energy = +8.1 kJ/mol
initiation entropy = -23.4 J/K mol
initiation enthalpy = +0.8 kJ/mol
- Based on data from H. Allawai et. al, “Thermodynamics and NMR of Internal G-T Mismatches in DNA” Biochemistry 36, 10581-10594 (1997).


















Right here is the right blog for everyone who wants to understand this topic. You understand so much its almost hard to argue with you (not that I actually will need to…HaHa). You certainly put a brand new spin on a subject which has been written about for years. Wonderful stuff, just excellent!
LikeLike