NMR samples were made using an OpenTron (OT2) liquid handler by adding 20 microliters of AcAc into a deuterated NMR solvent (typically 0.6 mL). The NMR solvent often contains 0.03% v/v tetramethylsilane (TMS), which is using as an internal reference. These solutions were pipetted into a 3 mm NMR tube and flame sealed. A rack of flame sealed NMR samples are shown below, along with the bottle of AcAc and 20 microliters in one of the OT2 pipette tips.

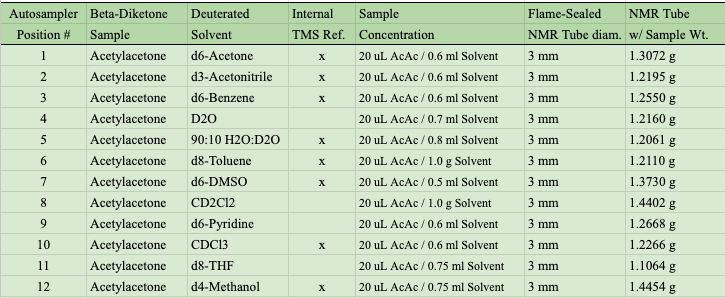
Here is a summary of the NMR samples:
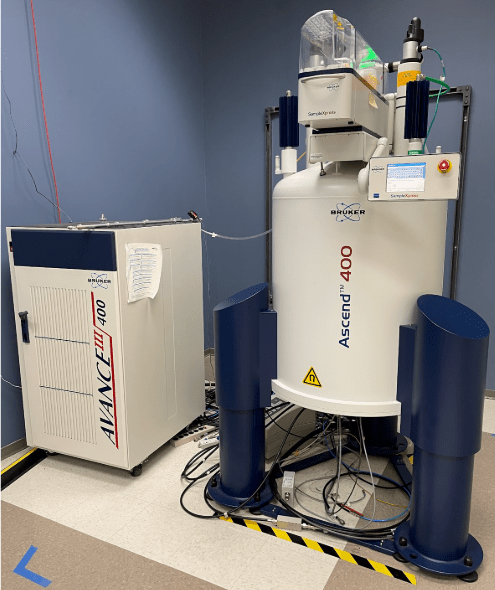
These samples are put into the SampleXpress autosampler on one of the Bruker NMR systems at ASU (ISTB1 L2-63). Below is an image of the Bruker 400 MHz NMR system
These samples can be remotely setup in a queue for automated NMR and the resulting NMR data is available at spintropy.com (in the ASU_CHM343 user). For example, these samples were setup to run a standard 1H (8 scans with 60 sec delay at 298K) NMR experiment on the Bruker 500 MHz NMR system and below is a screenshot of the ‘Data’ tab in the ASU_CHM343 spintropy account:

Students can login to the ASU_CHM343 spintropy account (password provided on Canvas) and the data can be downloaded (down arrow icon) or automatically opened in NMRium (spectrum icon). The filename indicates what deuterated solvent for each sample. This information is also given in the file information parameters. The downloaded data sets (in zip format) will also be put on the CHM343 GitHub repo for easy public access. As an example, a set of NMR data collected on the remote automated Bruker 500 MHz NMR of AcetylAcetone (AcAC) in several deuterated solvents (e.g., Acetone, ACN, Benzene, CD2Cl2, D2O, DMSO, ….) that are available on both Spintropy and GitHub, and have been linked to below for download (NMR Data collected Sept 25, 2025):
It is also common to look at the temperature dependence of tautomer equilibrium. Collecting the NMR at several different temperatures is often done so that the Van’t Hoff equation can be used to determine the change in Enthalpy and Entropy. As an example, a set of NMR data collected on the remote automated Bruker 400 MHz NMR of AcetylAcetone (AcAC) in d6-Benzene (C6D6) with TMS has been collected at several temperatures (e.g., 25 C, 35 C, 45 C, 55 C and 65 C) are available on GitHub, and have been linked to below for download:
The primary focus of NMR experiments on AcAc is typically elucidation of chemical equilibrium. However, it can also be important to consider the kinetics of chemical equilibria. In most solvents the kinetics of thermal equilibra of Keto-Enol tautomers in AcAc is fast relative to the timescale for NMR measurements (1-2 minutes). However, in some solvents the process is slow and the kintetics can be measured using NMR. As an example, a set of NMR data collected on the remote automated Bruker 400 MHz NMR of AcetylAcetone (AcAC) in d6-DMSO with TMS has been collected at several times after a temperature jump from 25 C to 65 C (e.g., 11 min, 13 min, …):
The remote NMR instruments allow the temperature to be set to specific values. However, it is common to use a standard to check the instruments temperature calibration. A common standard used is the splitting difference in d4-Methanol. A set of NMR data collected on the same remote automated Bruker 400 MHz NMR of d4-Methanol has been collected at several temperature and can be used to check the temperature calibration.